
Email : info@gghospital.in | Phone : +91 99622 29940
TEST FOR OVARIAN & BREAST CANCER
Home » TEST FOR OVARIAN AND BREAST CANCER
Most women who get breast cancer do not have any family history of breast cancer. Just because a family member had breast cancer does not always mean that you will get breast cancer.
We do know that there are some genes associated with a known increased risk of breast cancer. These are BRCA 1 (breast cancer 1, early onset human tumor suppressor gene) and BRCA 2 (breast cancer type 2, susceptibility protein). Only 10 percent of women with breast cancer have these inherited genes. These women usually get breast cancer at a young age and have multiple family members with breast or ovarian cancer.
Fewer than 3 out of every 100 breast cancers (3%) are caused by an inherited faulty gene. There are a group of genes that increase the risk of breast cancer and can be tested for, a few enlisted below are –
If you are known to have a gene that increases breast cancer risk, your specialist may suggest that you have regular screening.
Between 45 and 90 women out of 100 (45 to 90%) with a faulty BRCA1 or BRCA2 gene develop breast cancer during their lifetime. Faulty BRCA1 and 2 genes are rare. But they can also increase the risk of other cancers such as ovarian cancer, pancreatic and prostate cancer.
Faults in TP53 and PTEN mutations are rarer than BRCA1 and BRCA2 mutations. The TP53 gene normally controls when a cell divides. It is a type of tumor suppressor gene. It causes breast cancer as part of a rare cancer syndrome called Li Fraumeni syndrome. It causes less than 1 in 100 (1%) of all breast cancers diagnosed. PTEN is the gene fault in a rare condition called Cowden syndrome. It increases your risk of breast cancer. Advances in molecular genetics have led to the identification of numerous genes associated with inherited susceptibility to breast and/or ovarian cancer. Approximately 5-10% of all cases of breast cancer and ~25% of all cases of ovarian cancer are associated with a cancer predisposition gene.
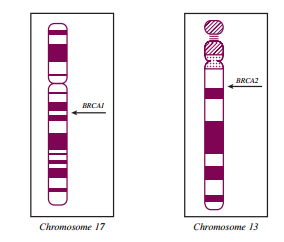
Most BRCA1 and BRCA2 mutations are predicted to produce a truncated protein product, and thus loss of protein function, although some missense mutations cause loss of function without truncation. Because inherited breast/ovarian cancer is an autosomal dominant condition, persons with a BRCA1 or BRCA2 mutation on one copy of chromosome 17 or 13 also carry a normal allele on the other paired chromosome. In most breast and ovarian cancers that have been studied from mutation carriers, deletion of the normal allele results in loss of all function, leading to the classification of BRCA1 and BRCA2 as tumor suppressor genes. In addition to, and as part of, their roles as tumor suppressor genes, BRCA1 and BRCA2 are involved in myriad functions within cells, including homologous DNA repair, genomic stability, transcriptional regulation, protein ubiquitination, chromatin remodeling, and cell cycle control.
Venkataraman AR: Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108 (2): 171- 82, 2002.
Narod SA, Foulkes WD: BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 4 (9): 665-76, 2004.
Development of a treatment plan that is likely to reduce cancer risk in individuals who test positive. This may include facilitating difficult medical decisions, like whether or not to have prophylactic mastectomy or oophorectomy.
BRCA1/2 testing maybe considered for individuals with a personal or family history of any of the following:

The following individuals have an increased risk for hereditary breast and ovarian cancer and are appropriate candidates for BRCA1/2 testing.
In some cases, limited family history may not allow for evaluation of these criteria. Limited family history is typically defined as <2 first- or second-degree female relatives living beyond 45 years on the same side of the family.*Close blood relatives include first, second and third-degree relatives on the same side of the family
